Projects in the Beeler Group are focused on synthesis and medicinal chemistry of biologically active small molecules. We focus on developing efficient and scalable processes to synthesize scaffolds of interest. We select molecules that we believe may be optimized as powerful tools to better understand biological processes important to human health. Ultimately, these tools can serve to identify new therapeutic targets or even be lead molecules for future therapeutics.
Photochemistry & Flow Chemistry
This area focuses on photochemical transformations toward the synthesis of natural products, natural product scaffolds, and other complex chemotypes of interest to medicinal chemistry and chemical biology. Flow chemistry has emerged as a powerful tool with many advantages over batch chemistry; its advantages are proved most dramatically in photochemistry, where steady state conditions can be scaled indefinitely. In our Group, we use photochemistry and flow chemistry in a complementary manner to develop robust, useful methods of accessing molecular scaffolds which would be otherwise evasive.
Organic Photoredox Catalysis - Synthesis of Classical Lignan Scaffolds
|
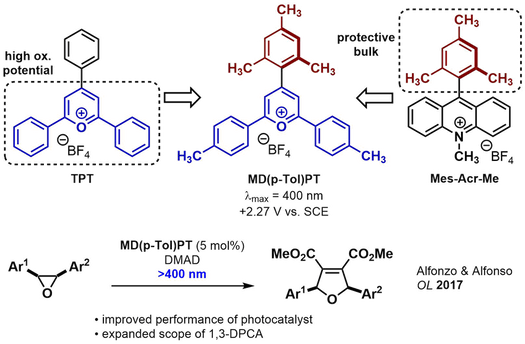
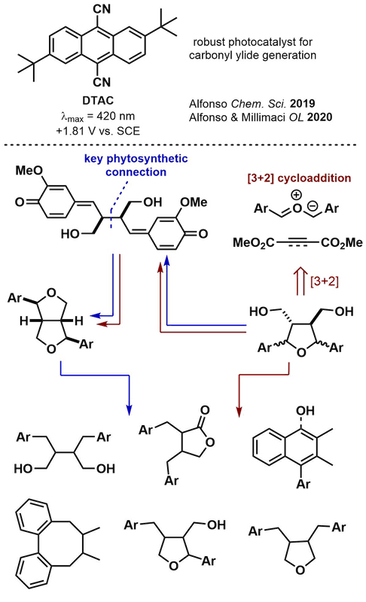
Classical lignan natural products (CLs) constitute a large, diverse family of phenylpropanoid dimers which exhibit interesting biological activity (e.g. podophyllotoxin). By employing improved organic photoredox catalysts - MD(p-Tol)PT and DTAC - we have gained coordinated access to all CL subtypes using 3+2 cycloadditions of carbonyl ylides and dipolarophiles. The postulated intermediate (bis para-quinone methide) mimics Nature's construction of the CL family and gives access to key entrypoints. Further manipulation of oxidation state grants access to the remaining CLs and several natural products with high efficiency - including the aryltetralin subfamily [link].
|
2+2 Photocycloadditions
|
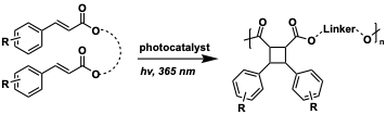
Truxinates are head-to-head cinnamic acid photodimers expressed broadly in Plantae and are found in a number of bioactive natural products. The robust and diastereoselective synthesis of truxinates has remained a major challenge since their isolation. Using filtered UV-light (>305 nm) and a bis-thiourea H-bonding catalyst in continuous flow, we have improved the yield and diastereoselectivity of the intermolecular [2+2] photocycloaddition of cinnamic esters [link]. With further development, we found that the use of biphasic slug flow dramatically accelerates the homodimerization by improving mixing and efficiency of irradiation. This improved protocol boosted yields, shortened reaction times, and expanded the reaction scope [link].
More recently we have found that catechol is a highly practical template for synthesizing homo- and heterodimeric beta truxinates in scalable fashion. We applied this strategy firstly to the synthesis of potent anticancer compounds, piperarborenines C, D, and E on multigram scale (currently in ChemRxiv; [doi]). A medicinal chemistry campaign on the beta piperarborenines is currently underway, as well as a full investigation of the scope and applications of the catechol-tethered truxinate synthesis. |
Currently, we have ongoing methodology efforts to selectively synthesize truxillic acids via an intramolecular tethering approach. We are also looking to investigate an intermolecular approach to truxillates utilizing pi-pi stacking interactions. Our work will expand synthetic approaches to unique cyclobutane derivatives.
|
Dearomative Ring Expansions
|
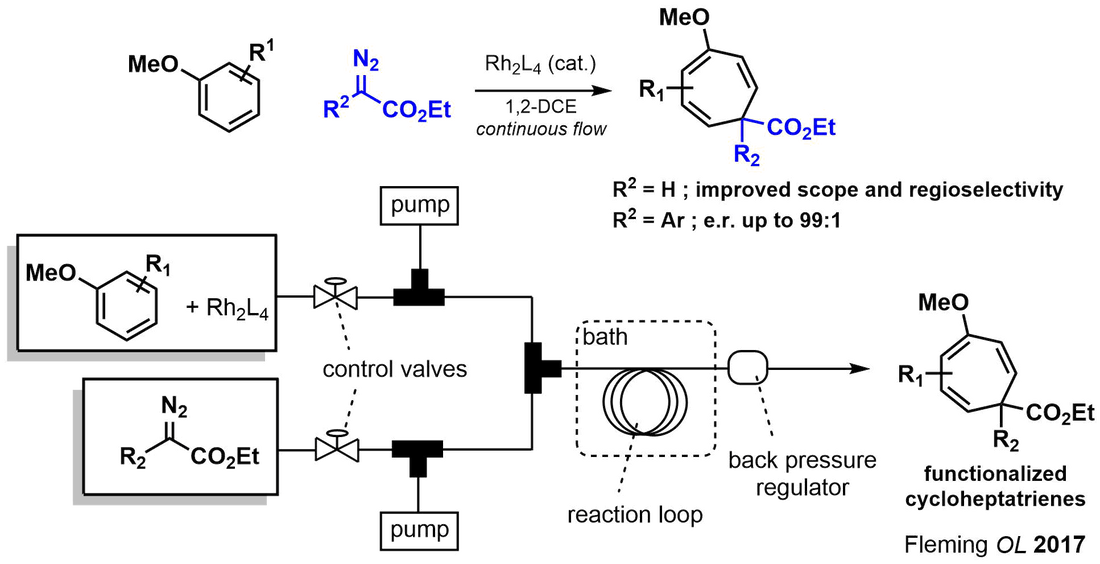
The intermolecular Buchner ring expansion is one of the few methods for generating seven-membered carbocycles from arenes. However historically, it has been burdened by mixtures of regioisomeric products, due in part to its exothermic nature and nitrogen out-gassing. By leveraging the back-pressure and increased heat exchange characteristic of flow reactors, we developed a method for the intermolecular Buchner ring expansion with improved regioselectivity and enantioselectivity. [link]
|
Azepines are represented broadly in natural products, pharmaceuticals, and investigational molecules. Traditional methods of azepine synthesis involve ring-forming reactions which can have significant limitations or rely on expensive transition metal catalysts (e.g. ring-closing metathesis). In our continued interest in dearomative ring expansion methodology, we developed a mild and efficient protocol wherein azepines are synthesized by a visible-light-mediated ring expansion from aromatic N-ylides, constituting a formal aza-Buchner ring expansion. On deprotonation with base under blue light irradiation, N-aromatic salts undergo a dearomative ring expansion providing monocyclic and polycyclic azepines. There are many established methods to synthesize pyridines, so this approach offers a convenient new route to access azepines by two-step N-alkylation followed by ring expansion. [link]
|
Development of Fluorescent Probes for Spatial Proteomics
|
Proteins are fundamental to cellular function, and their expression, location, and abundance are key to identification between a healthy and diseased cell. We develop state-of-the-art fluorescent probes capable of tagging proteins to quantify their localization within cells. In collaboration with a multidisciplinary team, we aim to identify and map proteome architectures, allowing scientists to determine how disease and aging effect protein networks in cells.
|
FLOW CHEMISTRY
One of the core components in our research is development of continuous flow technologies to facilitate the synthesis and medicinal chemistry of target molecules. Flow chemistry has emerged as a powerful tool to enable reactions that have traditionally been challenging to carry our and/or difficult to scale. We are utilizing flow technologies to develop photochemical reactions and reactions utilizing reactive intermediates. The methods we develop are applied to the synthesis of molecules for medicinal chemistry.
Flow Synthesis on the International Space Station
|
|
The ability to synthesize organic chemicals on-demand may be crucial in the future of space travel and colonization. We worked closely with Space Tango to develop a Flow Chemistry Cube Lab for conducting organic chemistry reactions in a self-contained system on the International Space Station National Lab (ISS-NL). A first of its kind, the Cube Lab is equipped with cameras, pumps, flow meters, pressure sensors, and sophisticated valving to ensure safe and efficient operation. On its first mission (SpaceX CRS-20), the Cube Lab carried out three different chemical reactions each with multiple trials. This has served as an important proof of concept for synthesis-on-demand onboard the ISS-NL and its findings will guide future efforts for organic synthesis in spaceflight.
|
Polymerization in Flow
|
Cyclobutane Polymers in Continuous Flow
Despite the extensive literature surrounding [2+2] photocycloaddition, published synthetic strategies for the corresponding [2+2] photopolymerization to prepare cyclobutane polymers (CBPs) are limited in scope and number. Herein, we demonstrate an improved and efficient synthetic method to prepare well-defined, structurally complex cyclobutane polymers via solution state [2+2] photopolymerization in continuous flow. Biodegradable Polymers in Flow
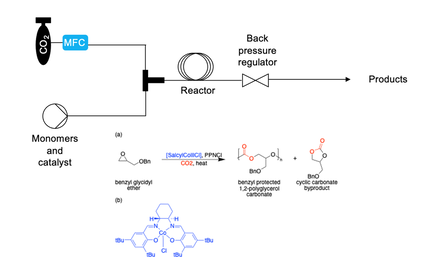
Viscoelastic materials synthesized from carbon dioxide constitute a large, diverse family of polymers that are biodegradable, inexpensive, and non-toxic. We aim to access more facile and reliable routes to access these polymers utilizing flow chemistry. We are also interested in tuning their monomer’s properties to increase adhesive and cohesive strength and alter glass transition temperatures. |
Linear and Cyclic Polymers in Continuous Flow
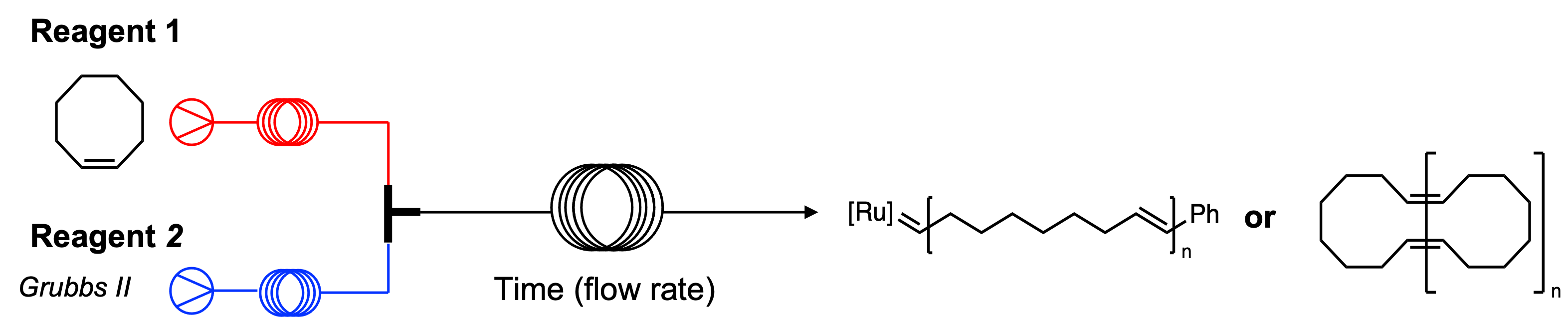
Cyclic polymers are valuable to numerous industries including the biomedical sector due to their unique topology. Similarly, linear polymers are often the target products for industrial applications. Both cyclic and linear polymers can be synthesized via the Ring Opening Metathesis Polymerization (ROMP), however methods to selectively synthesize one polymeric product are limited. We propose the selective synthesis of both cyclic and linear polymers via continuous flow. |
HIGH THROUGHPUT EXPERIMENTATION
|
|
High-throughput experimentation (HTE) is a set of technologies that allow for many hundreds or thousands of reaction conditions to be assessed simultaneously. Naturally, HTE has proven itself tremendously useful in organic chemistry, being instrumental in the efficient assembly of large medicinal chemistry libraries and in identifying optimal reaction conditions for process optimization. In the Beeler Lab, we’re using HTE to produce highly-consistent datasets to train machine learning algorithms on chemical reactivity. To do this, we’ve built an automation-friendly platform for the distribution of starting materials, incubation of reactions, and have developed a simple, ultra-rapid sample analysis technology to measure reaction success. Working closely with the Kolaczyk Group, we’re taking a multi-faceted approach toward quantifying our understanding on what intrinsic and extrinsic variables affect conversion to product and how best to train machines on such phenomena.
|
MEDICINAL CHEMISTRY
Using Peptides for the Inhibition of Protein-Protein Interactions
|
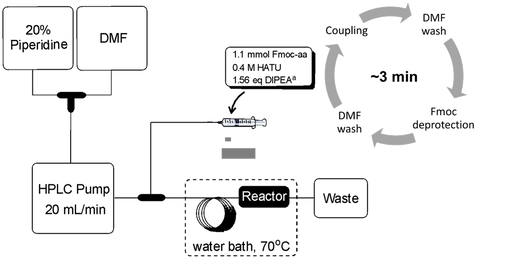
A vast majority of disease-associated biological activities involves interactions between two or more proteins (PPIs). PPIs are involved in several diseases including cancer, diabetes, and neurological disorders. In several cases, discovery of small molecule drugs for PPIs has been challenging because PPIs have larger surfaces with several low affinity binding spots scattered on the protein surface rather than a single hydrophobic pocket. By using peptides, we are able to reach these binding spots to potentially inhibit specific PPIs. By using synthetic peptides, we are able to use unnatural amino acids, peptidomimetic bonds, and novel cyclization methods that can enhance the peptides utilizing structural motifs that are not possible with current recombinant methods.
|
Our lab’s research involve the use of both small molecules as well as peptides for the inhibition of PPIs. Through fast flow peptide synthesis, we are able to produce peptides at a significantly shorter time than the conventional batch synthesis. Areas of research include: Acute kidney injury via modulation of the interaction between Nucleophosmin and Bcl-Associated X protein, and inhibition of the interaction between KEAP1 with the transcription factor Nrf2, a validated target for inflammation and neurodegenerative diseases.
|
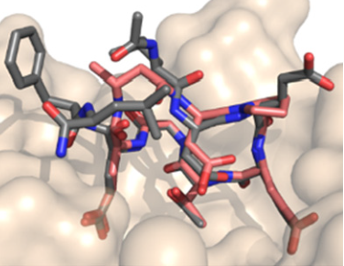
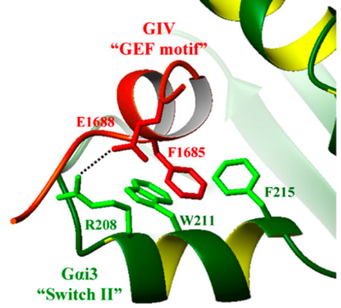
Small Molecule Inhibitors for the Disruption of GIV-Gai3 Interaction
|
Heterotrimeric G proteins are key molecular switches that turn on intracellular signaling cascades that control cell behavior. This trimer is composed of three subunits, α, β, and γ, and are canonically activated by G protein-coupled receptors (GPCRs). Proteins, like Guanine nucleotide Exchange Factors (GEFs) exist intracellularly and act as ligands to activate G proteins. One such non-receptor GEF is Girdin (GIV), which plays an important role in cell migration by its interaction with the enhancement of Akt activation and actin cytoskeleton remodeling. Our collaborators have determined GIV binds to Gαi3 as part of the signaling for cell migration and cancer cell metathesis. In a collaborative effort, we screened a large library of small molecules identifying disruptors of the GIV-Gαi interface. Through these efforts, we envision designing first-in-class small molecule modulators for the disruption of this interface as a potential therapeutic for metastatic cancers.
|
Our research focuses on using medicinal chemistry techniques to synthesize small molecules as potential inhibitors of this PPI to be assayed for their activity and used to develop a structure activity relationship model that will facilitate further optimization. We aim to use small molecule scaffolds as a tool to establish the binding site by synthesizing small molecule photo-affinity probes which will assist in rational design.
|
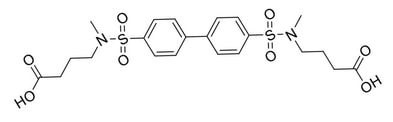
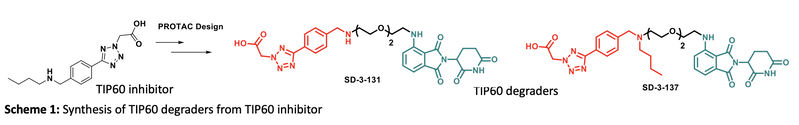
The Ubiquitin-Mediated Protein Degradation of Histone Acetyltransferase TIP60 to Suppress Treg Cell Activity
|
|
Regulatory T (Treg) cells suppress inflammatory immune responses. Treg cell over-expression has been observed in many cancers, making it a great target for potential cancer therapies. A key Treg transcription factor, Foxp3, is a protein that is acetylated by TIP60 histone acetyltransferase protein in multiple types of cancers. Even though Foxp3 acetylation by TIP60 has been targeted for over a decade, there have been no successful inhibitors with high potency and selectivity. Therefore, we are using the targeted protein degradation technology for faster and more beneficial ubiquitination and degradation of TIP60. Protein degradation is a relatively new strategy where the target protein is being degraded by the proteasomal degradation machinery inside the cells. This approach has more benefits compared to functional inhibition, including a longer lasting suppression of the target protein, higher suppression rate of the protein, and more effective cell proliferation and higher apoptosis induction. We have synthesized a small library of TIP60 inhibitors through a fragment based design, and the most active molecule was tested in a Treg suppression assay by our collaborator- Wayne Hancock at Philadelphia Children’s hospital. Using rational design, we optimized the potency of existing TIP60 inhibitors and generated a library of CRBN-based degraders by conjugating the active molecules CRBN ligands. By varying the linker composition and length, we were able to assess conditions that allow for effective degradation of TIP60 in vitro and in vivo.
|
CATALYSIS
Vanadium(V) Catalytic Aerobic Oxidations
|
|
A new air-stable catalyst for the oxidative dehydrogenation of benzylic alcohols under ambient conditions has been developed. The synthesis and characterization of this compound and the related monomeric and dimeric V(IV)- and V(V)-pinF (pinF = perfluoropinacolate) complexes were reported in 2020. An ongoing collaboration with the Doerrer Group at Boston University focuses on mechanism elucidation and understanding the full scope of reactivity.
|