Beeler Group Publications
Publications:
58. "Predicting Emission Wavelengths in Benzobisoxazole-Based OLEDs with Gradient Boosted Ensemble Models" Shambhavi Tannir, Yuning Pan, Nathaniel Josephs, Christopher Cunningham, Nathan R. Hendrick, Annie Beckett, James McNeely, Aaron Beeler, Malika Jeffries-EL*, and Eric D. Kolaczyk* J. Phys. Chem. A 2024. [Link]
|
We demonstrate the use of gradient-boosted ensemble models that accurately predict emission wavelengths in benzobis[1,2-d:4,5-d′]oxazole (BBO) based fluorescent emitters. We have curated a database of 50 molecules from previously published data by the Jeffries-EL group using density functional theory (DFT) computed ground and excited state features. We consider two machine learning (ML) models based on (i) whole cruciform molecules and (ii) their constituent fragment molecules. Both ML models provide accurate predictions with root-mean-square errors between 30 and 36 nm, competitive with state-of-the-art deep learning models trained on orders of magnitude more molecules, and this accuracy holds even when tested on four new BBO emitters unseen by the models. We also provide an interpretable feature importance analysis and discuss the relevant relationships between DFT and changes in predicted emission wavelength.
|
57. "Regioselective Photoredox Catalyzed Cycloadditions of Acyclic Carbonyl Ylides" Alexandra M. Millimaci, Antonin C. Knirsch, and Aaron B. Beeler* ChemRxiv. 2024. doi:10.26434/chemrxiv-2024-k035t
|
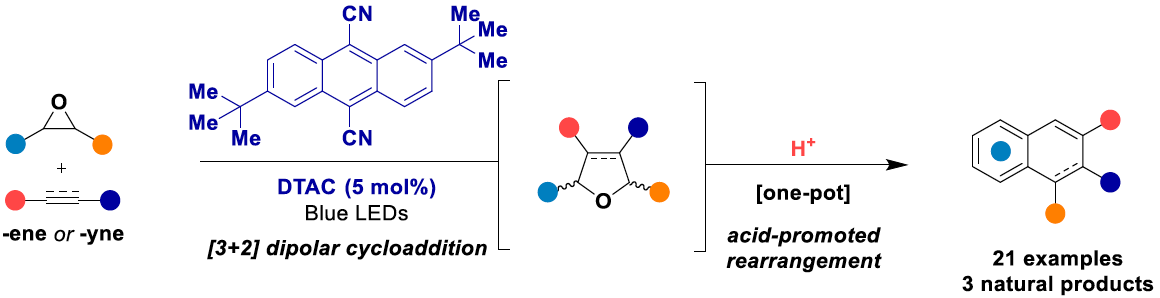
A photoredox catalyzed [3+2] dipolar cycloaddition between acyclic carbonyl ylides generated from 𝛼-cyano epoxides and dipolarophiles is described. This method, influenced by anionic charge localization and temperature control, enabled the synthesis of regioselective functionalized cyclic ethers. By leveraging different dipolarophiles, Lewis acid mediated activation afforded either furan or hydroxy-dihydronaphthalene scaffolds. A direct synthesis of lignan natural products isodiphyllin and diphyllin is achieved by exploiting the nitrile’s reactivity as a directing handle for the desired regioisomer.
|
56. "Sigma Receptor Ligands Are Potent Antiprion Compounds that Act Independently of Sigma Receptor Binding" Robert C. C. Mercer, Nhat T. T. Le, Douglas G. Fraser, Mei C. Q. Houser, Aaron B. Beeler, and David A. Harris*. ACS Chem. Neurosci. 2024. [Link]
|
Prion diseases are invariably fatal neurodegenerative diseases of humans and other animals for which there are no effective treatment options. Previous work from our laboratory identified phenethylpiperidines as a novel class of anti-prion compounds. While working to identify the molecular target(s) of these molecules, we unexpectedly discovered ten novel antiprion compounds based on their known ability to bind to the sigma receptors, σ1R and σ2R, which are currently being tested as therapeutic or diagnostic targets for cancer and neuropsychiatric disorders. Surprisingly, however, knockout of the respective genes encoding σ1R and σ2R (Sigmar1 and Tmem97) in prion-infected N2a cells did not alter the antiprion activity of these compounds, demonstrating that these receptors are not the direct targets responsible for the antiprion effects of their ligands. Further investigation of the most potent molecules established that they are efficacious against multiple prion strains and protect against downstream prion-mediated synaptotoxicity. While the precise details of the mechanism of action of these molecules remain to be determined, the present work forms the basis for further investigation of these compounds in preclinical studies. Given the therapeutic utility of several of the tested compounds, including rimcazole and haloperidol for neuropsychiatric conditions, (+)-pentazocine for neuropathic pain, and the ongoing clinical trials of SA 4503 and ANAVEX2-73 for ischemic stroke and Alzheimer’s disease, respectively, this work has immediate implications for the treatment of human prion disease.
|
55. "Accessing Cyclobutane Polymers: Overcoming Synthetic Challenges via Efficient Continuous Flow [2 + 2] Photopolymerization" Sara El-Arid, Jason M. Lenihan, Andrew Jacobsen, Aaron B. Beeler* and Mark W. Grinstaff*. ACS Macro Lett. 2024 [Link]
|
We report an improved and efficient method to prepare well-defined, structurally complex truxinate cyclobutane polymers via a thioxanthone sensitized solution state [2 + 2] photopolymerization. Monomers with varying electron density and structure polymerize in good to excellent yields to afford a library of 42 polyesters. Monomers with internal olefin separation distances of greater than 5 Å undergo polymerization via intermolecular [2 + 2] photocycloaddition readily, as opposed to the intramolecular [2 + 2] photocycloaddition observed in monomers with olefins in closer proximity. Use of a continuous flow reactor decreases reaction time, increases polymer molecular weight, and decreases dispersity compared to batch reactions. Furthermore, under continuous flow, polymerization is readily scalable beyond what is possible with batch reactions. This advancement ushers truxinate cyclobutane-based polyesters, which have been historically limited to a few examples and only research scale quantities, to the forefront of development as new materials for potential use across industry sectors.
|
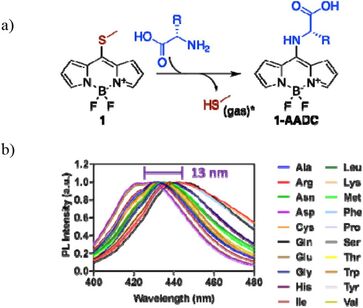
54. "Development of BODIPY-based Fluorescent Probes for Highly Selective Amino Acid Identification" Nathaniel Hendrick, Andrew Martin, Andrew Chan, Jason Lenihan, Harrison Reiter, Mitchell Clough, Lian Jiang, Eddie Whaley, Yoo Jeong Huh, James McNeely, Anderson Chen, Andrew Emili, and Aaron Beeler. bioRxiv 2024.04.02.587799 [link]
|
Amino acid sensing is a powerful tool for intra- and extra-cellular assays. We report herein the generation and optimization of bioconjugatable fluorescent reporter for pan-amino acid sensing, the OMNI probe, that through inductive electronics produces a unique photophysical response upon coupling to the α-amino group of an amino acid depending on the identity of the adjacent side chain. Using an auto-photocatalyzed Meerwein arylation reaction, we created a library of bis-arylated 8-methylthio-BODIPY dye derivatives exhibiting improved spectral variation, brightness, dynamic range, robustness, water solubility, and reactivity. Aryl groups with optimal photophysical behaviors were combined to create asymmetric versions conferring high spectral range, brightness, and water solubility. Combining aryl group modifications yielded a set of engineered asymmetric probes that achieved complete spectral resolution in a multi-variable analysis (fluorescent emission, lifetime, brightness) for all twenty native amino acid-dye conjugates while increasing reactivity toward amino acids in aqueous buffer. With our robust OMNI probe design, pan-amino acid resolution and identification through high sensitivity fluorescence down to single molecules is now routinely possible for diverse potential applications including protein sequencing.
|
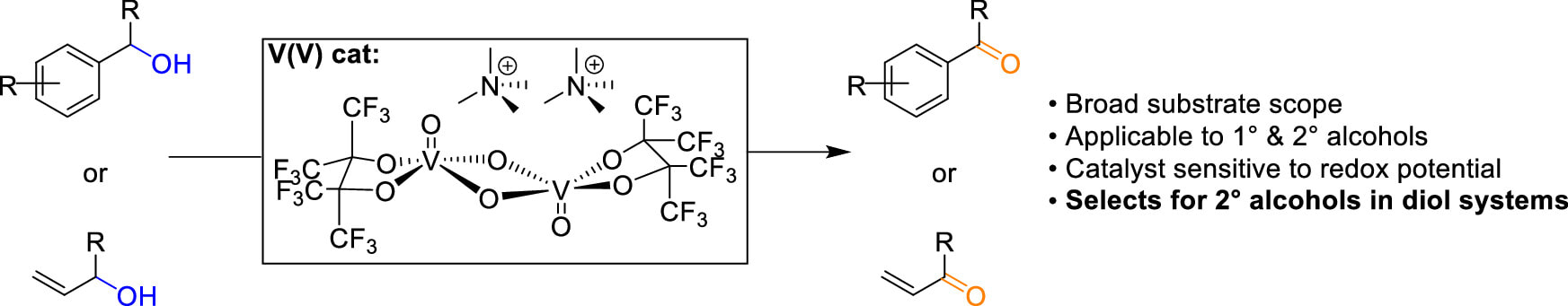
53. "Chemoselective Aerobic Oxidation of Alcohols Utilizing a Vanadium(V) Catalyst" Maggie I. Kitt, Emily Amir, Eleanor R. Sloane, Douglas G. Fraser, John E. Cerritelli, Caroline S. M. Sabanos, James H. McNeely, John K. Snyder, Linda H. Doerrer, and Aaron B. Beeler. ACS Catal. 2024. [link]
|
Work remains to develop an aerobic catalyst that will selectively oxidize secondary alcohols in the presence of primary alcohols. In principle, this is possible when there is an oxidation potential preference for doing so. One potential strategy is the use of a low oxidation potential catalyst. Recently, we reported a dimeric vanadium(V)–(μ-O)2 perfluoropinacolate (pinF) complex with the ability to oxidize alcohols via dehydrogenation that was sensitive to the alcohol redox potential. Herein, we report the scope, chemoselectivity, and insights into the mechanism by which V(V) cat performs oxidations on activated primary and secondary alcohols.
|
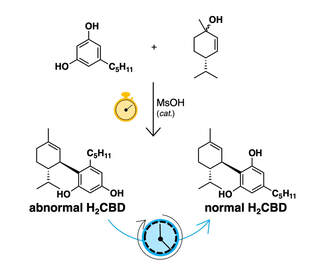
52. "Synthesis of Neocannabinoids Using Controlled Friedel-Crafts Reactions" Alexandra M. Millimaci, Richard V. Trilles, James H. McNeely , Lauren E. Brown, Aaron B. Beeler, and John A. Porco Jr. J. Org. Chem. 2023. [link]
|
A one-step transformation to produce 8,9-dihydrocannabidiol (H2CBD) and related “neocannabinoids” via controlled Friedel–Crafts reactions is reported. Experimental and computational studies probing the mechanism of neocannabinoid synthesis from cyclic allylic alcohol and substituted resorcinol reaction partners provide understanding of the kinetic and thermodynamic factors driving regioselectivity for the reaction. Herein, we present the reaction scope for neocannabinoid synthesis including the production of both normal and abnormal isomers under both kinetic and thermodynamic control. Discovery and optimization of this one-step protocol between various allylic alcohols and resorcinol derivatives are discussed and supported with density functional theory calculations.
|
51. "Small Molecule targeting of GPCR-independent noncanonical G-protein signaling in cancer" Jingyi Zhao and Vincent DiGiacomo and Mariola Ferreras-Gutierrez and Shiva Dastjerdi and Alain Ibáñez de Opakua and Jong-Chan Park and Alex Luebbers and Qingyan Chen and Aaron Beeler and Francisco J. Blanco and Mikel Garcia-Marcos. Proceedings of the National Academy of Sciences. 2023. [link]
Activation of heterotrimeric G-proteins (Gαβγ) by G-protein-coupled receptors (GPCRs) is a quintessential mechanism of cell signaling widely targeted by clinically-approved drugs. However, it has become evident that heterotrimeric G-proteins can also be activated via GPCR-independent mechanisms that remain untapped as pharmacological targets. GIV/Girdin has emerged as a prototypical non-GPCR activator of G proteins that promotes cancer metastasis. Here, we introduce IGGi-11, a first-in-class smallmolecule inhibitor of non-canonical activation of heterotrimeric G-protein signaling. IGGi-11 binding to G-protein α-subunits (Gαi) specifically disrupted their engagement with GIV/Girdin, thereby blocking non-canonical G-protein signaling in tumor cells, and inhibiting pro-invasive traits of metastatic cancer cells in vitro and in mice. In contrast, IGGi-11 did not interfere with canonical G-protein signaling mechanisms triggered by GPCRs. By revealing that small molecules can selectively disable non-canonical mechanisms of G-protein activation dysregulated in disease, these findings warrant the exploration of therapeutic modalities in G-protein signaling that go beyond targeting GPCRs.
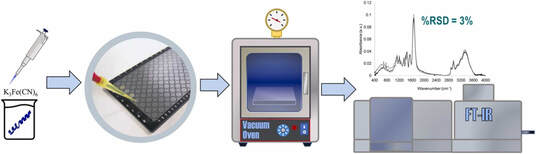
50. "High-Throughput Infrared Spectroscopy for Quantification of Peptides in Drug Discovery" Nathaniel Hendrick, Douglas Fraser, Raffeal Bennett, Kaitlyn Corazzata, Donovon A. Adpressa, Alexey A. Makarov, Aaron Beeler. Journal of Pharmaceutical and Biomedical Analysis. 2023, 115350. [link]
|
Peptides have gained an increasing importance in drug discovery as potential therapeutics. Discovery efforts toward finding new, efficacious peptide-based therapeutics have increased the throughput of peptide development, allowing the rapid generation of unique and pure peptide samples. However, high-throughput analysis of peptides may be still challenging and can encumber a high-throughput drug discovery campaign. We report herein a fit-for-purpose method to quantify peptide concentrations using high-throughput infrared spectroscopy (HT-IR). Through the development of this method, multiple critical method parameters were optimized including solvent composition, droplet deposition size, plate drying procedures, sample concentration, and internal standard. The relative absorbance of the amide region (1600-1750 cm-1) to the internal standard, K3Fe(CN)6 (2140 cm-1), was determined to be most effective at providing lowest interference for measuring peptide concentration. The best sample deposition was achieved by dissolving samples in a 50:50 v/v allyl alcohol/water mixture. The developed method was used on 96-well plates and analyzed at a rate of 22 minutes per plate. Calibration curves to measure sample concentration versus response relationship displayed sufficient linearity (R2 > 0.95). The repeatability and scope of detection was demonstrated with eighteen peptide samples that were measured with most values below 20% relative standard deviation. The linear dynamic range of the method was determined to be between 1 and 5 mg/mL. This developed HT-IR methodology could be a useful tool in peptide drug candidate lead identification and optimization processes.
|
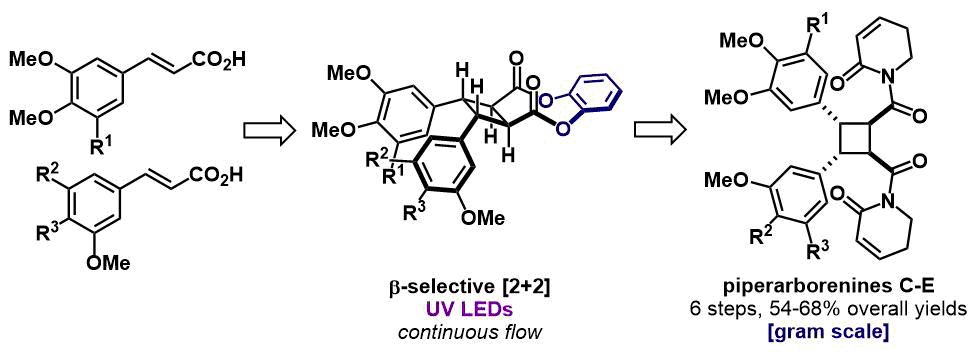
49. "Multigram Scale Synthesis of Piperarborenines C-E" Jason M. Lenihan, Matthew J. Mailloux, Aaron B. Beeler. Org. Process Res. Dev. 2022, 26, 1812-1819. [link]
|
We report the multigram scale synthesis of heterodimeric β-truxinic imides piperarborenines C-E using a catechol-tethered diastereoselective intramolecular [2+2] photocycloaddition. Key innovations lie in the use of catechol as a practical auxiliary for the synthesis of homo- and heterodimeric β-truxinates, and the use of a UV-LED flow photoreactor in the [2+2] step. This approach is highly scalable, requiring a single column purification, no photocatalysts, and no cryogenic conditions.
|
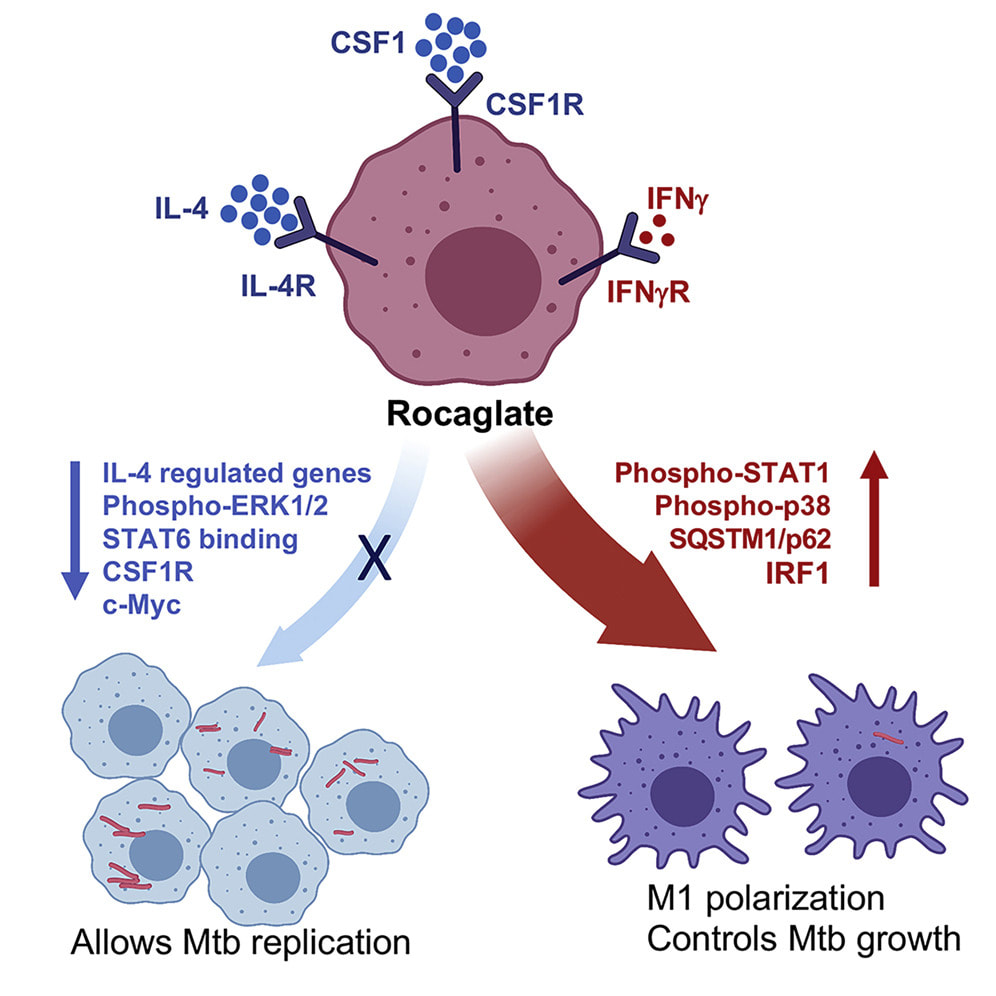
48. "Channeling macrophage polarization by rocaglates increases macrophage resistance to Mycobacterium tuberculosis" Sujoy Chatterjee, Shivraj M. Yabaji, Oleksii S. Rukhlenko, Bidisha Bhattacharya, Emily Waligurski, Nandini Vallavoju, Somak Ray, Boris N. Kholodenko, Lauren E. Brown, Aaron B. Beeler, Alexander R. Ivanov, Lester Kobzik, John A. Porco, Igor Kramnik. iScience, 2021, 102845. [Link]
|
Macrophages contribute to host immunity and tissue homeostasis via alternative activation programs. M1-like macrophages control intracellular bacterial pathogens and tumor progression. In contrast, M2-like macrophages shape reparative microenvironments that can be conducive for pathogen survival or tumor growth. An imbalance of these macrophages phenotypes may perpetuate sites of chronic unresolved inflammation, such as infectious granulomas and solid tumors.
We have found that plant-derived and synthetic rocaglates sensitize macrophages to low concentrations of the M1-inducing cytokine IFN-gamma and inhibit their responsiveness to IL-4, a prototypical activator of the M2-like phenotype. Treatement of primary macrophages with rocaglates enhanced phagosome - lysosme fusion and control of intracellular mycobacteria. Thus, rocaglates represent a novel class of immunomodulators that can direct macrophage polarization towards the M1-like phenotype in complex microenvironments associated with hypofunction of type 1 and/or hyperactivation of type 2 immunity, e.g. chronic bacterial infections, allergies and, possibly, certain tumors. |
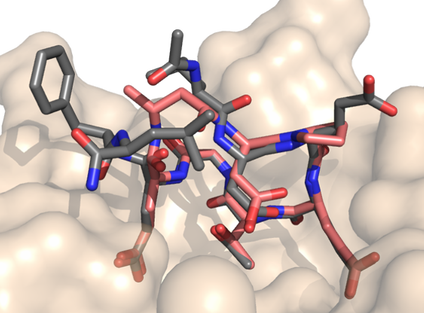
47. "Recapitulating the Binding Affinity of Nrf2 for KEAP1 in a Cyclic Heptapeptide, Guided by NMR, X-Ray Crystallography, and Machine Learning" Paula C. Ortet, Samantha N. Muellers, Lauren A. Viarengo-Baker, Kristina Streu, Blair R. Szymczyna, Aaron B. Beeler, Karen N. Allen, and Adrian Whitty. JACS 2021, 143, 3779. [Link]
|
Macrocycles, including macrocyclic peptides, have shown promise for targeting challenging protein-protein interactions (PPIs). One PPI of high interest is between Kelch-like ECH-Associated Protein-1 (KEAP1) and Nuclear Factor (Erythroid-derived 2)-like 2 (Nrf2). Guided by X-ray crystallography, NMR, modeling, and machine learning, we show that the full 20 nM binding affinity of Nrf2 for KEAP1 can be recapitulated in a cyclic 7-mer peptide, c[(D)-β-homoAla-DPETGE]. This compound was identified from the Nrf2-derived linear peptide GDEETGE (KD = 4.3 mM) solely by optimizing the conformation of the cyclic compound, without changing any KEAP1 interacting residue. X-ray crystal structures were determined for each linear and cyclic peptide variant bound to KEAP1. Despite large variations in affinity, no obvious differences in the conformation of the peptide binding residues or in the interactions they made with KEAP1 were observed. However, analysis of the X-ray structures by machine learning showed that locations of strain in the bound ligand could be identified through patterns of sub-Ångstrom distortions from the geometry observed for unstrained linear peptides. We show that optimizing the cyclic peptide affinity was driven partly through conformational pre-organization associated with a proline substitution at position 78 and with the geometry of the non-interacting residue Asp77, and partly by decreasing strain in the ETGE motif itself. This approach may have utility in dissecting the trade-off between conformational pre-organization and strain in other ligand-receptor systems. We also identify a pair of conserved hydrophobic residues flanking the core DxETGE motif which play a conformational role in facilitating the high-affinity binding of Nrf2 to KEAP1.
|
46. "Unified Synthesis of Azepines by Visible-Light-Mediated Dearomative Ring-Expansion of Aromatic N-Ylides" Matthew J. Mailloux, Gabrielle S. Fleming, Shruti S. Kumta, and Aaron B. Beeler Org. Lett. 2021, 23, 525. [Link]
Featured in: Nöel et al Chem. Rev. 2022, 122, 2752-2906. [doi] ; Sarpong & Levin et al Nature Synthesis 2022 ; [doi]
Featured in: Nöel et al Chem. Rev. 2022, 122, 2752-2906. [doi] ; Sarpong & Levin et al Nature Synthesis 2022 ; [doi]
|
|
Herein we report a unified approach to azepines by dearomative photochemical rearrangement of aromatic N-ylides. Deprotonation of quaternary aromatic salts with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or N,N,N’,N’-tetramethylquanidine (TMG) under visible light irradiation provides mono- and polycyclic azepines in yields up to 98%. This ring-expansion presents a new mode of access to functionalized azepines from N-heteroarenes using two straightforward steps and simple starting materials.
|
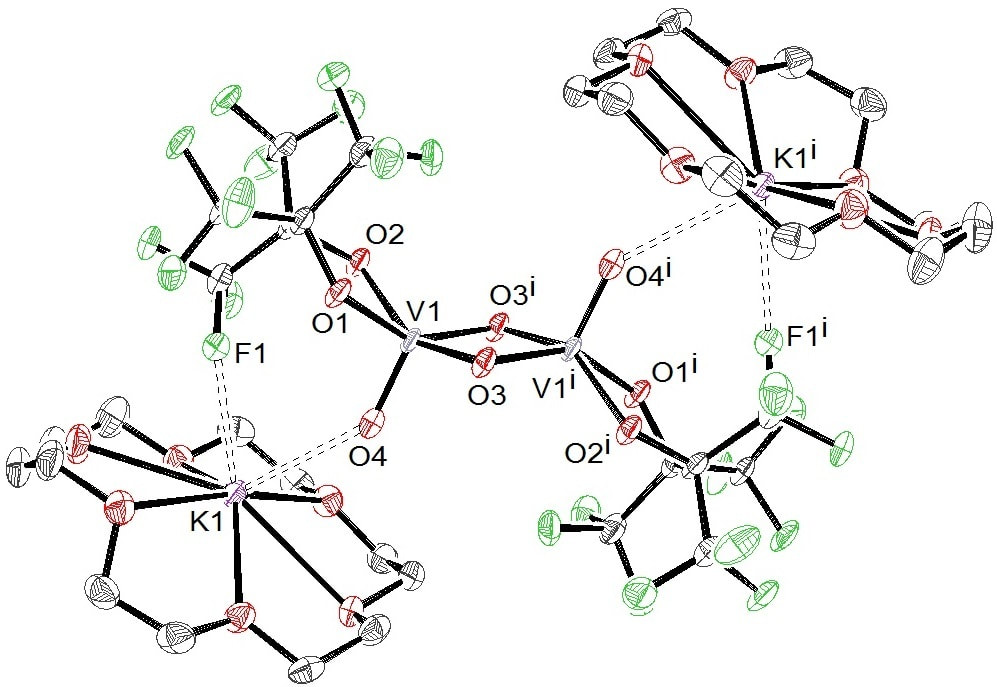
45. "Reversible PCET and Ambient Catalytic Oxidative Alcohol Dehydrogenation by {V=O} Perfluoropinacolate Complexes" Jessica K. Elinburg, Samantha L. Carter, Joshua J. M. Nelson, Douglas G. Fraser, Michael P. Crockett, Aaron B. Beeler, Ebbe Nordlander, Arnold L. Rheingold, and Linda H. Doerrer. Inorg. Chem. 2020, 59, 22, 16500–16513 [Link]
|
A new air-stable catalyst for the oxidative dehydrogenation of benzylic alcohols under ambient conditions has been developed. The synthesis and characterization of this compound and the related monomeric and dimeric V(IV)- and V(V)-pinF (pinF = perfluoropinacolate) complexes are reported herein. Monomeric V(IV) complex (Me4N)2[V(O)(pinF)2] (1) and dimeric (μ-O)2- bridged V(V) complex (Me4N)2[V2(O)2(μ-O)2(pinF)2] (3a) are prepared in water under ambient conditions. Monomeric V(V) complex (Me4N)[V(O)(pinF)2] (2) may be generated via chemical oxidation of 1 under an inert atmosphere, but dimerizes to 3a upon exposure to air. Complexes 1 and 2 display a perfectly reversible VIV/V couple at 20 mV (vs Ag/AgNO3), whereas a quasi-reversible VIV/V couple at −865 mV is found for 3a. Stoichiometric reactions of 3a with both fluorenol and TEMPOH result in the formation of (Me4N)2[V2(O)2(μ-OH)2(pinF)2] (4a), which contains two V(IV) centers that display antiferromagnetic coupling. In order to structurally characterize the dinuclear anion of 4a, {K(18C6)}+ countercations were employed, which formed stabilizing K···O interactions between the counterion and each terminal oxo moiety and H-bonding between the oxygen atoms of the crown ether and μ-OH bridges of the dimer, resulting in {K(18C6)}2[V2(O)2(μ- OH)2(pinF)2] (4b). The formal storage of H2 in 4a is reversible and proton-coupled electron transfer (PCET) from crystals of 4a regenerates 3a upon exposure to air over the course of several days. Furthermore, the reaction of 3a (2%) under ambient conditions with excess fluorenol, cinnamyl alcohol, or benzyl alcohol resulted in the selective formation of fluorenone (82% conversion), cinnamaldehyde (40%), or benzaldehyde (7%), respectively, reproducing oxidative alcohol dehydrogenation (OAD) chemistry known for VOx surfaces and demonstrating, in air, the thermodynamically challenging selective oxidation of alcohols to aldehydes/ketones.
|
44. "Photoredox Generated Carbonyl Ylides Enable a Modular Approach to Aryltetralin, Dihydronaphthalene and Arylnaphthalene Lignans" Alfonzo, E.; Millimaci, A. M.; Beeler, A. B. Org. Lett. 2020, 22, 6489. [Link]
Featured in: Overman & Pitre Chem. Rev. 2022, 22, 1717-1751. ; [doi]
Featured in: Overman & Pitre Chem. Rev. 2022, 22, 1717-1751. ; [doi]
|
A one-pot synthesis of dihydronaphthalenes and arylnaphthalenes from epoxides and common dipolarophiles is described. The reaction proceeds through photoredox activation of epoxides to carbonyl ylides, which undergo concerted [3 + 2] dipolar cycloaddition with dipolarophiles to provide tetrahydrofurans or 2,5-dihydrofurans. In the same flask, acid promoted rearrangement affords densely functionalized dihydronaphthalenes and arylnaphthalenes, respectively, in an overall redox-neutral sequence of transformations. Succinct total synthesis (4−6 steps) of pycnanthulignene B and C and justicidin E are reported.
|
43. "A sterically encumbered photoredox catalyst enables the unified synthesis of the classical lignan family of natural products" Alfonzo, E.; Beeler, A. B. Chem. Sci. 2019, Advance Article. [link]
Featured in: Overman & Pitre Chem. Rev. 2022, 22, 1717-1751. ; [doi]
Featured in: Overman & Pitre Chem. Rev. 2022, 22, 1717-1751. ; [doi]
|
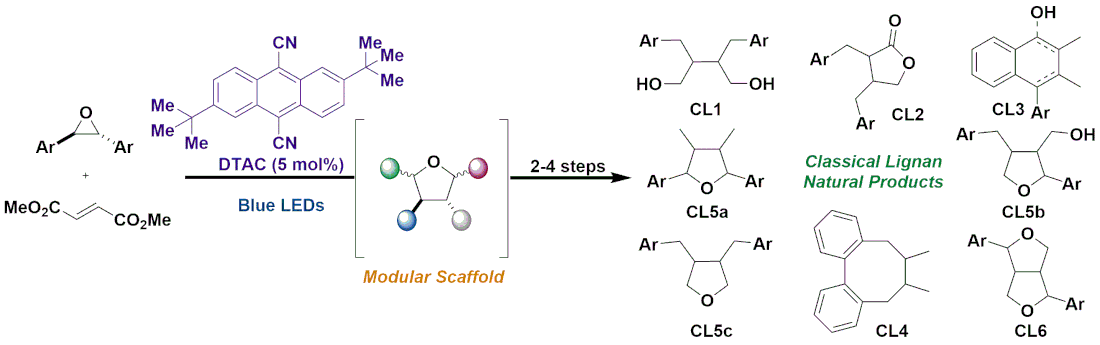
Herein, we detail a unified synthetic approach to the classical lignan family of natural products that hinges on divergence from a common intermediate that was strategically identified from nature's biosynthetic blueprints. Efforts toward accessing the common intermediate through a convergent and modular approach resulted in the discovery of a sterically encumbered photoredox catalyst that can selectively generate carbonyl ylides from electron-rich epoxides. These can undergo concerted [3 + 2] dipolar cycloadditions to afford tetrahydrofurans, which were advanced (2–4 steps) to at least one representative natural product or natural product scaffold within all six subtypes in classical lignans. The application of those synthetic blueprints to the synthesis of heterolignans bearing unnatural functionality was demonstrated, which establishes the potential of this strategy to accelerate structure–activityrelationship studies of these natural product frameworks and their rich biological activity.
|
42. "One-pot Synthesis of Epoxides from Benzyl Alcohols and Aldehydes" Alfonzo, E.; Mendoza, J. W. L.; Beeler, A. B. Bel. J. Org. Chem. 2018, 14, 2308-2312. [link]
|
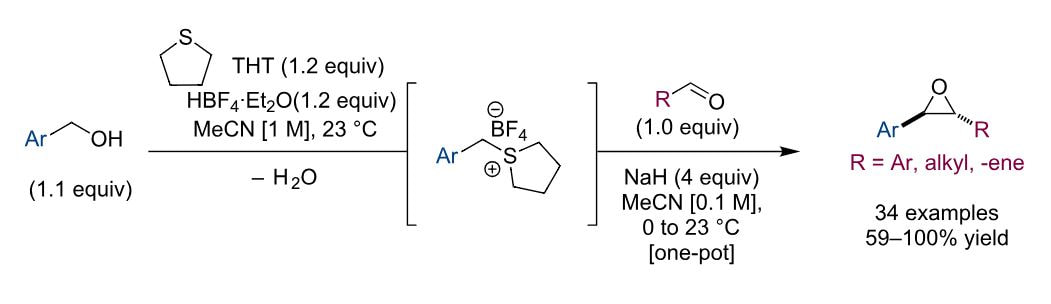
A one-pot synthesis of epoxides from commercially available benzyl alcohols and aldehydes is described. The reaction proceeds through in situ generation of sulfonium salts from benzyl alcohols and their subsequent deprotonation for use in Corey-Chaykovsky epoxidation of aldehydes. The generality of the method is exemplified by the synthesis of 34 epoxides that were made from an array of electronically and sterically varied alcohols and aldehydes.
|
41. "Synthesis of Complex Stereoheptads En Route to Daphnane Diterpene Orthoesters" Nguyen, L. V.; Beeler, A. B. Org. Lett. 2018, 20, 5177-5180. [link]
|
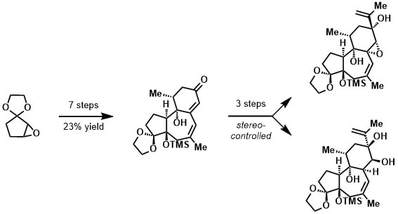
Tricyclic cores of the daphnane diterpene orthoesters (DDOs) are synthesized in ten steps from readily available materials. Key to their assembly is the development of a stereocontrolled p-quinol functionalization sequence which enables rapid access to DDO C-ring stereopolyads from simple precursors. Problems encountered in stereo- and regioselectivity are highlighted and solved by exact changes in choreography although it is shown the undesired stereochemical outcomes also proceed with high selectivity.
|
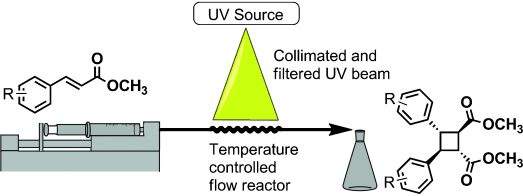
40. "Liquid-Liquid Slug Flow Accelerated [2+2] Photocycloaddition of Cinnamates" Telmesani, R.; White, J. A. H.; Beeler, A. B. Chem. Photo. Chem. 2018, 2, 1-6. [link]
|
|
[2+2] photocycloaddition of cinnamates using a previously described cone reactor and a bis-thiourea catalyst is greatly accelerated by employing liquid-liquid slug flow. In most cases, a 4-fold acceleration in reaction time was observed with equivalent or superior yields. This approach has enabled significant improvement in the reaction throughput and expansion of the substrate scope to include challenging substrates. It has also enabled, to our knowledge, the first reported direct photodimerization in solution of electron rich cinnamamide substrates.
|
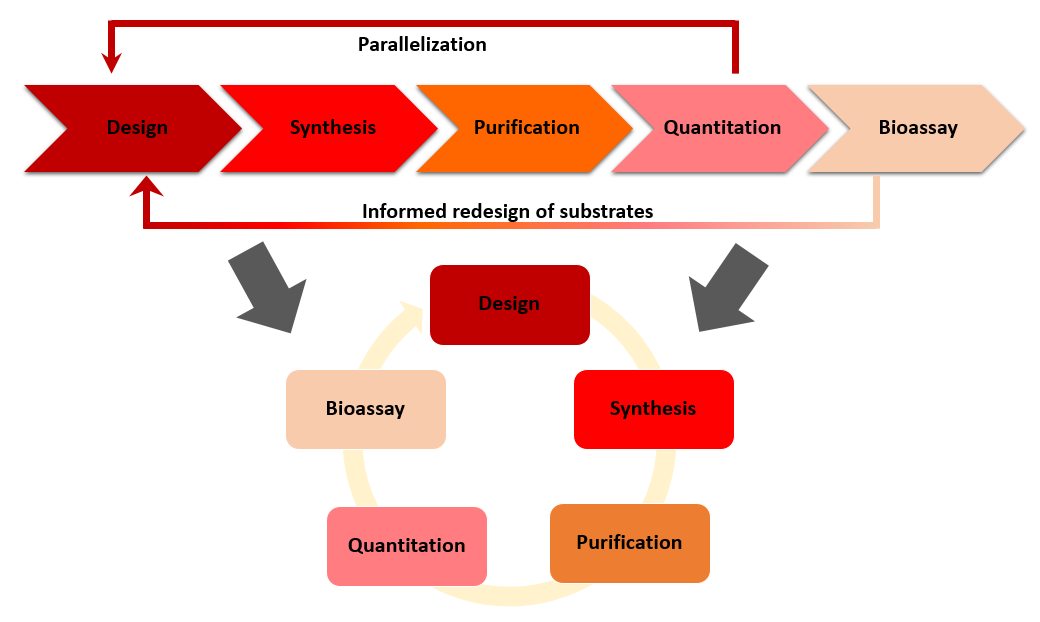
39. "Integrated Drug Discovery in Continuous Flow" Fleming, G. S.; Beeler, A. B. J. Flow Chem. 2017, 7, 124. (Invited Review) [link]
|
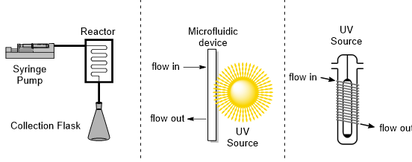
There are great opportunities for innovation in the drug discovery process, particularly in the lead development phase. The traditional “design–synthesize–screen” cycle has seen little innovation as a whole despite major advances at each stage, including automated purification and synthesis as well as high throughput biological screening. It could be argued that the hit-to-lead and lead optimization processes remain slow and modular with inefficient flow of information, resulting in a loss of time and money. New flow technologies may provide a promising foundation for developing a continuous integrated small molecule optimization platform that would greatly enhance hit-to-lead and lead optimization programs. Herein, we discuss major developments in integrating synthesis, purification, screening, and machine learning into a single continuous-flow platform and provide some insight into future directions of this field.
|
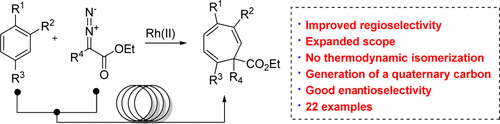
38. "Regioselective and Enantioselective Intermolecular Buchner Ring Expansions in Flow" Fleming, G. S.; Beeler, A. B. Org. Lett. 2017, 19, 5268. [link]
Highlighted in Organic Process Research and Development "Highlights from the Literature," December 2017 [link]
Highlighted in Organic Process Research and Development "Highlights from the Literature," December 2017 [link]
|
The first example of a regioselective and enantioselective intermolecular Buchner ring expansion is reported using continuous flow. The practicality and scope of the reaction are greatly improved under flow conditions. Reactions of ethyl diazoacetate with symmetric and nonsymmetric arenes afford cycloheptatrienes in good yield and excellent regioselectivity. The first example of an asymmetric intermolecular Buchner reaction is demonstrated with disubstituted diazo esters in good to excellent enantioselectivity. The asymmetric reactions proceed with absolute regioselectivity to afford cycloheptatrienes with an all-carbon quaternary center.
|
37. "A Photochemical Flow Reactor for Large Scale Syntheses of Aglain and Rocaglate Natural Product Analogs" Yueh, H.; Gao, Q.; Porco, J. A.; Beeler, A. B. Bioorg. Med. Chem. 2017, 25, 6197. [link]
|
Herein, we report the development of continuous flow photoreactors for large scale ESIPT-mediated [3+2]-photocycloaddition of 2-(p-methoxyphenyl)-3-hydroxyflavone and cinnamate- derived dipolarophiles. These reactors can be efficiently numbered up to increase throughput two orders of magnitude greater than the corresponding batch reactions.
|
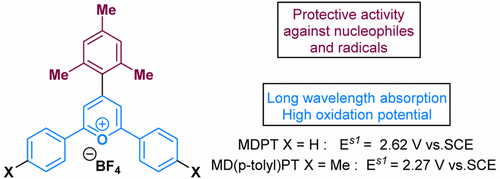
36. "Redesign of a Pyrylium Photoredox Catalyst and Its Application to the Generation of Carbonyl Ylides" Alfonzo, E.; Alfonso, F. S.; Beeler, A. B. Org. Lett. 2017, 19, 2989-2992. [link]
|
We report the exploration into photoredox generation of carbonyl ylides from benzylic epoxides using newly designed 4-mesityl-2,6-diphenylpyrylium tetrafluoroborate (MDPT) and 4-mesityl-2,6-di-p-tolylpyrylium tetrafluoroborate (MD(p-tolyl)PT) catalysts. These catalysts are excited at visible wavelengths, are highly robust, and exhibit some of the highest oxidation potentials reported. Their utility was demonstrated in the mild and efficient generation of carbonyl ylides from benzylic epoxides that otherwise could not be carried out by current common photoredox catalysts.
|
35. "Identification of Anti-Prion Compounds Using a Novel Cellular Assay" Imberdis, T.; Heeres, J. T.; Yueh, H.; Fang, C. Zhen, J.; Rich, C. B.; Glicksman, M.; Beeler, A.; Harris, D. A. J. Bio. Chem. 2016, 291, 26164-26176. [link]
|
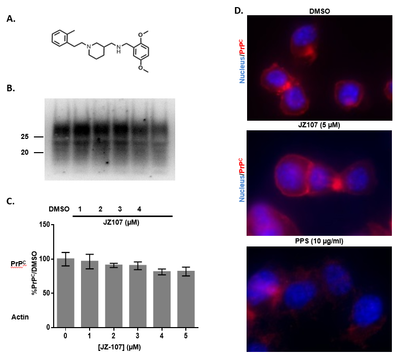
Prion diseases are devastating neurodegenerative disorders with no known cure. One strategy for developing therapies for these diseases is to identify compounds that block conversion of the cellular form of the prion protein (PrPC) into the infectious isoform (PrPSc). Most previous efforts to discover such molecules by high-throughput screening methods have utilized, as a read-out, a single kind of cellular assay system: neuroblastoma cells that are persistently infected with scrapie prions. Here, we describe the use of an alternative cellular assay based on suppressing the spontaneous cytotoxicity of a mutant form of PrP (Δ105–125). Using this assay, we screened 75,000 compounds, and identified a group of phenethyl piperidines (exemplified by LD7), which reduces the accumulation of PrPSc in infected neuroblastoma cells by >90% at low micromolar doses, and inhibits PrPSc-induced synaptotoxicity in hippocampal neurons. By analyzing the structure-activity relationships of 35 chemical derivatives, we defined the pharmacophore of LD7, and identified a more potent derivative. Active compounds do not alter total or cell-surface levels of PrPC, and do not bind to recombinant PrP in surface plasmon resonance experiments, although at high concentrations they inhibit PrPSc-seeded conversion of recombinant PrP to a misfolded state in an in vitro reaction (RT-QuIC). This class of small molecules may provide valuable therapeutic leads, as well as chemical biological tools to identify cellular pathways underlying PrPSc metabolism and PrPC function.
|
34. "Introduction: Photochemistry in Organic Synthesis" Beeler, A. B. Chem. Rev. 2016, 116, 9629-9630. [link]
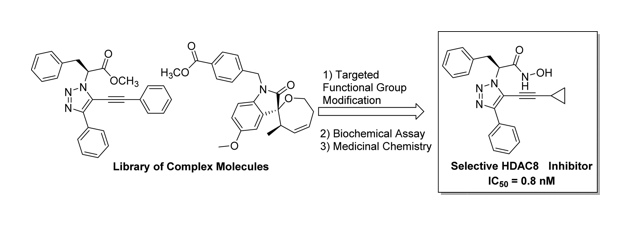
33. “Development of a Potent and Selective HDAC8 Inhibitor" Ingham, O. J.; Paranal, R. M.; Smith, W. B.; Escobar, R. A.; Yueh, H.; Snyder, T.; Porco, J. A.; Bradner, J. E.; Beeler, A. B. ACS Med. Chem. Lett. 2016, 7, 929. [link]
32. "Fine-tuning of macrophage activation using synthetic rocaglate derivatives" Bhattacharya, B.; Chatterjee, S.; Devine, W. G.; Kobsik, L.; Beeler, A. B.; Porco, J. A.; Kramnik, I. Scientific Reports 2016, 6, 24409. [link]
|
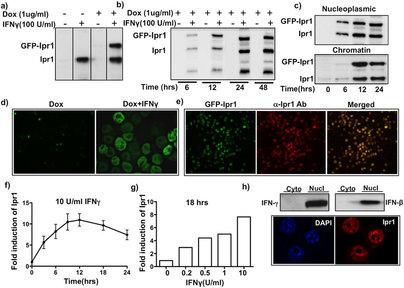
Drug-resistant bacteria represent a significant global threat. Given the dearth of new antibiotics, host-directed therapies (HDTs) are especially desirable. As IFN-gamma (IFNγ) plays a central role in host resistance to intracellular bacteria, including Mycobacterium tuberculosis, we searched for small molecules to augment the IFNγ response in macrophages. Using an interferon-inducible nuclear protein Ipr1 as a biomarker of macrophage activation, we performed a high-throughput screen and identified molecules that synergized with low concentration of IFNγ. Several active compounds belonged to the flavagline (rocaglate) family. In primary macrophages a subset of rocaglates 1) synergized with low concentrations of IFNγ in stimulating expression of a subset of IFN-inducible genes, including a key regulator of the IFNγ network, Irf1; 2) suppressed the expression of inducible nitric oxide synthase and type I IFN and 3) induced autophagy. These compounds may represent a basis for macrophage-directed therapies that fine-tune macrophage effector functions to combat intracellular pathogens and reduce inflammatory tissue damage. These therapies would be especially relevant to fighting drug-resistant pathogens, where improving host immunity may prove to be the ultimate resource.
|
31. "Photochemistry in Flow" Beeler, A. B.; Corning, S. in Photochemistry: Volume 43; Royal Society of Chemistry: Cambridge, 2016.
30. “[2+2]-Photocycloaddition of Cinnamates in Flow and Development of a Thiourea Catalyst” Telmesani, R.; Park, S. H.; Lynch-Colameta, T.; Beeler, A. B. Angew. Chem. Int. Ed. 2015, 54, 11521-11525. [link]
Highlighted in Organic Process Research and Development "Highlights from the Literature," September 2015 [link]
Highlighted in Organic Process Research and Development "Highlights from the Literature," September 2015 [link]
|
Cyclobutanes derived from the dimerization of cinnamic acids are the core scaffolds of many molecules with potentially interesting biological activities. By utilizing a powerful flow photochemistry platform developed in our laboratory, we have evaluated the effects of flow on the dimerization of a range of cinnamate substrates. During the course of the study we also identified a bis(thiourea) catalyst that facilitates better reactivity and moderate diastereoselectivity in the reaction. Overall, we show that carrying out the reaction in flow in the presence of the catalyst affords consistent formation of predictable cyclobutane diastereomers.
|
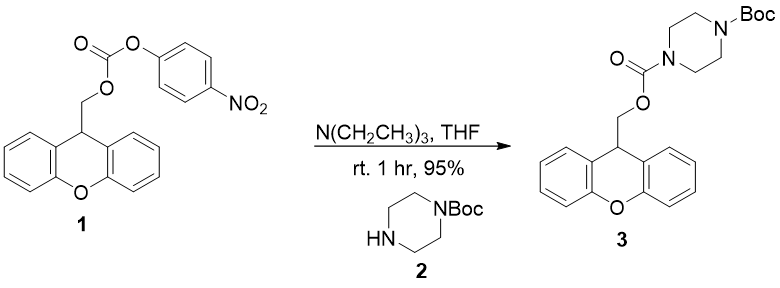
29. “Development of a Photolabile Amine Protecting Group Suitable for Multistep Flow Synthesis” Yueh, H.; Voevodin, A.; Beeler, A. B. J. Flow Chem. 2015, 5, 155-159. [link]
|
9-Hydroxymethylxanthene derivatives were optimized as a photolabile protecting group for amines in flow chemistry. 9-Methylxanthene and 2-methoxy-9-methylxanthene showed excellent deprotection yields in protic and aprotic solvents, respectively. The protecting group has good stability in acidic, basic, and thermal conditions and was successfully utilized for protection and deprotection of a variety of amines. A multistep continuous-flow synthesis of a piperazinylcarbonyl-piperidine derivative utilized the 2-methoxy-9-methylxanthene as the key protecting group utilized in an orthogonal manner.
|
28. “Multidimensional Reaction Screening for Photochemical Transformations as a Tool for Discovering New Chemotypes” Martin, M. I.; Goodell, J. R.; Ingham, O. J.; Porco, J. A.; Beeler. A. B. J. Org. Chem. 2014, 79, 3838-3846. [link]
- Highlighted in Derek Lowe’s blog ‘In the Pipeline’ [link].
|
We have developed an automated photochemical microfluidics platform that integrates a 1 kW high-pressure Hg vapor lamp and allows for analytical pulse flow or preparative continuous flow reactions. Herein, we will discuss the use of this platform toward the discovery of new chemotypes through multidimensional reaction screening. We will highlight the ability to discretely control wavelengths with optical filters, allowing for control of reaction outcomes.
|
Publications through 2012
27. “Synthesis of azaphilone-based chemical libraries, Achard, M.; Beeler, A.; Porco, J., ACS Comb. Sci. 2012, 14, 236-280. [link]
26. “Synthesis and Reactivity of Bicyclo[3.2.1]Octanoid-Derived Cyclopropanes,” Goodell, J. R.; Poole, J. L.; Beeler, A. B.; Aubé, J.; Porco, J. A. Jr. J. Org. Chem. 2011, 76, 9792. [link]
25. “Development of a Photochemical Microfluidics Platform,” Pimparkar, K.; Yen, B.; Goodell, J. R.; Martin, V. I.; Lee, W-H.; Porco, J. A. Jr.; Beeler, A. B. Jensen, K. F. J. Flow Chem. 2011, 2, 53. [link]
24. “Remodelling of the Natural Product Fumagillol Employing a Reaction Discovery Approach,” Balthaser, B. R.; Maloney, M. C.; Beeler, A. B.; Porco, J. A. Jr.; Snyder, J. K. Nature Chem. 2011, 3, 969. [link]
23. “Truncated Aspidosperma Alkaloid-like Scaffolds: Unique Structures For the Discovery of New, Bioactive Compounds,” Benson, S. C.; Lee, L.; Wei, W.; Ni, F.; David, J.; Olmos, J.; Strom, K. R.; Beeler, A. B.; Cheng, K. C-C.; Inglese, J.; Kota, S.; Takahashi, V.; Strosberg, A. D.; Connor, J. H.; Bushkin, G. G.; Snyder, J. K. Heterocycles, 2011, 84, 135. [link]
22. “Discovery of New Antimalarial Chemotypes Through Chemical Methodology and Library Development,” Brown, L. E.; Cheng, K, C-C; Wei, W-G.; Yuana, P.; Dai, P.; Trilles, R.; Ni, F.; Yuan, J.; MacArthur, R.; Guhab, R.; Johnson, R. L.; Suc, X-Z; Dominguez, M. M.; Snyder, J. K.; Beeler, A. B.; Schaus, S. E.; Inglese, J.; Porco, J. A. Jr. Proc. Nat. Aca. Sci. 2011, 108, 6775. [link]
21. “Catalytic Enantioselective Alkylative Dearomatization−Annulation: Total Synthesis and Absolute Configuration Assignment of Hyperibone K,” Qi, J.; Beeler, A. B.; Zhang, Q.; Porco, J. A. Jr. J. Am. Chem. Soc. 2011, 132, 13642. [link]
20. “Multidimensional Screening and Methodology Development for Condensations Involving Complex 1,2-Diketones,” Goodell, J. R.; Leng, B.; Snyder, T. K.; Beeler, A. B.; Porco, J. A. Jr. Synthesis, 2010, 2254. [link]
19. “Tandem Processes Identified from Reaction Screening: Nucleophilic Addition to Aryl N-Phosphinylimines Employing La(III)-TFAA Activation,” Kinoshita, H.; Ingham, Oscar, J.; Ong, W. W.; Beeler, A. B.; Porco, J. A., Jr. J. Am. Chem. Soc. 2010, 132, 6412. [link]
18. “A Time-Resolved Fluorescence–Resonance Energy Transfer Assay for Identifying Inhibitors of Hepatitis C Virus Core Dimerization,” Kota, S.; Scampavia, L.; Spicer, T.; Beeler, A. B.; Takahashi, V.; Snyder, J. K.; Porco, J. A., Jr.; Hodder, P.; Strosberg, A. D. Assay Drug Dev. 2010, 8, 96. [link]
17. “Development of an Automated Microfluidic Reaction Platform for Multidimensional Screening: Reaction Discovery Employing Bicyclo[3.2.1]octanoid Scaffolds.” Goodell J. R.; McMullen, J. P; Zaborenko, N.; Maloney, J. R.; C-X Ho; Jensen, K. F.; Porco, J. A. Jr.; Beeler, A. B. J. Org. Chem. 2009, 74, 6169. [link]
16. “Reaction Discovery Employing Macrocycles: Transannular Cyclizations of Macrocyclic Bis-lactams,” Han, C.; Rangarajan, S.; Voukides, A. C.; Beeler, A. B.; Johnson, R.; Porco, J. A., Jr. Org. Lett., 2009, 11, 413–416. [link]
15. “Library Synthesis Using 5,6,7,8-Tetrahydro-1,6-naphthyridines as Scaffolds,” Zhou, Y.; Beeler, A. B.; Cho, S.; Wang, Y.; Franzblau, S. G.; Snyder, J. K. J. Comb. Chem. 2008, 10, 534–540. [link]
14. “Identification of novel epoxide inhibitors of hepatitis C virus replication using a high-throughput screen,” Peng, L. F.; Kim, S. S.; Matchacheep, S.; Lei, X.; Su, S.; Lin, W.; Runguphan, W.; Choe, W-H; Sakamoto, N.; Ikeda, M.; Kato, N.; Beeler, A. B.; Porco, J. A. Jr.; Schreiber, S. L.; Chung, R. T. Antimicrobi. Agents Chemo. 2007, 10, 3756. [link]
13. “Nucleophilic addition to N-phosphinylimines by rare-earth-metal triflate/trifluoroacetic anhydride activation,” Winnie W. Ong; Aaron B. Beeler; Sarathy, Kesavan; James S. Panek; John A. Porco Angew. Chem. Int. Ed. 2007, 39, 7470. [link]
12. “1,2,3,4-Tetrahydro-1,5-naphthyridines and related heterocyclic scaffolds: exploration of suitable chemistry for library development,”Grace H. C. Woo; Aaron B. Beeler; John K. Snyder Tetrahedron 2007, 63, 5649. [link]
11. “Generation of Oxamic Acid Libraries: Antimalarials and Inhibitors of Plasmodium falciparum Lactate Dehydrogenase,” Choi, S-R.; Beeler, A. B.; Pradhan, A.; Watkins, E. B.; Rimoldi, J. M.; Tekwani, B.; Avery, M. A. J. Comb. Chem. 2007, 9, 292. [link]
10. “Discovery of Chemical Reactions through Multidimensional Screening,” Beeler, A. B.; Su, S.; Singleton, C. A.; Porco, J. A. Jr. J. Am. Chem. Soc. 2007, 129,1413. [link]
9. “Synthesis of 1,4,5-trisubstituted-1,2,3-triazoles by copper-catalyzed cycloaddition-coupling of azides and terminal alkynes,” Gerard, B; Ryan, J.; Beeler, A. B.; Porco, J. A Jr. Tetrahedron, 2006, 62, 6405. [link]
8. “Convergent Synthesis of Complex Diketopiperazines Derived from Pipecolic Acid Scaffolds and Parallel Screening against GPCR Targets,” Dandapani, S.; Lan, P.; Beeler, A. B.; Beischel, S.; Abbas, A.; Roth, B. L.; Porco, J. A. Jr.; Panek, J. S. J. Org. Chem. 2006, 71, 8934.[link]
7. “Synthesis of a Library of Complex Macrodiolides Employing Cyclodimerization of Hydroxy Esters,” Beeler, A. B.; Acquilano, D. E.; Su, Q.; Yan, F.; Roth, B. L.; Panek, J. S.; Porco, J. A. Jr. J. Comb. Chem. 2005, 7, 673. [link]
6. “Chemical Library Synthesis Using Convergent Approaches,” Beeler, A. B.; Schaus, S. E.; Porco, J. A. Jr. Curr. Opin. Chem. Bio. 2005, 9, 277. [link]
5. “Convergent Synthesis of a Complex Oxime Library Using Chemical Domain Shuffling,” Su S.; Eastwood, E. L.; Beeler, A. B.; Yeager, A. R.; Lan, P.; Arumugasamy, J.; Acquilano, D. E.; Min, G. K.; Giguere, J. R.; Lei, X.; Zhou, Y.; Panek, J. S.; Snyder, J. K.; Schaus, S. E.; Porco, J. A. Jr. Org. Lett. 2005, 9, 2751. [link]
4. “Synthesis and In-Vitro Biological Evaluation of Fluorosubstituted-4-Phenyl-1,2,3,6-Tetrahydropyridines as Monoamine Oxidase B Substrates,” Beeler, A. B.; Gadepalli, R. S.; Steyn, S.; Castagnoli, N. Jr.; Rimoldi, J. M. Biorg. Med. Chem. 2003, 11, 5229-5234. [link]
3. “Stereochemical Diversity Through Cyclodimerization: Synthesis of Polyketide-like Macrodiolides,” Su, Q.; Beeler, A. B.; Lobkovsky, E.; Porco, J. Jr.; Panek, J. Org. Lett. 2003, 5, 2149-2152. [link]
2. “Toxicity of Fipronil and Its Degradation Products in Procambarus sp.: Field and Laboratory Studies,” Schlenk, D.; Huggett, D. B.; Allgood, J.; Bennett, E.; Rimoldi, J.; Beeler, A. B.; Block, D.; Holder, A. W.; Hovinga, R.; Bedient, P.Arch. Environ. Contam. Toxicol. 2001, 41, 325-332. [link]
1. “Synthesis of fipronil sulfide, an active metabolite from the parent insecticide fipronil,” Beeler, A. B.; Schlenk, D. K.; Rimoldi, J. M. Tetrahedron Lett. 2001, 42, 5371-5372
26. “Synthesis and Reactivity of Bicyclo[3.2.1]Octanoid-Derived Cyclopropanes,” Goodell, J. R.; Poole, J. L.; Beeler, A. B.; Aubé, J.; Porco, J. A. Jr. J. Org. Chem. 2011, 76, 9792. [link]
25. “Development of a Photochemical Microfluidics Platform,” Pimparkar, K.; Yen, B.; Goodell, J. R.; Martin, V. I.; Lee, W-H.; Porco, J. A. Jr.; Beeler, A. B. Jensen, K. F. J. Flow Chem. 2011, 2, 53. [link]
24. “Remodelling of the Natural Product Fumagillol Employing a Reaction Discovery Approach,” Balthaser, B. R.; Maloney, M. C.; Beeler, A. B.; Porco, J. A. Jr.; Snyder, J. K. Nature Chem. 2011, 3, 969. [link]
23. “Truncated Aspidosperma Alkaloid-like Scaffolds: Unique Structures For the Discovery of New, Bioactive Compounds,” Benson, S. C.; Lee, L.; Wei, W.; Ni, F.; David, J.; Olmos, J.; Strom, K. R.; Beeler, A. B.; Cheng, K. C-C.; Inglese, J.; Kota, S.; Takahashi, V.; Strosberg, A. D.; Connor, J. H.; Bushkin, G. G.; Snyder, J. K. Heterocycles, 2011, 84, 135. [link]
22. “Discovery of New Antimalarial Chemotypes Through Chemical Methodology and Library Development,” Brown, L. E.; Cheng, K, C-C; Wei, W-G.; Yuana, P.; Dai, P.; Trilles, R.; Ni, F.; Yuan, J.; MacArthur, R.; Guhab, R.; Johnson, R. L.; Suc, X-Z; Dominguez, M. M.; Snyder, J. K.; Beeler, A. B.; Schaus, S. E.; Inglese, J.; Porco, J. A. Jr. Proc. Nat. Aca. Sci. 2011, 108, 6775. [link]
21. “Catalytic Enantioselective Alkylative Dearomatization−Annulation: Total Synthesis and Absolute Configuration Assignment of Hyperibone K,” Qi, J.; Beeler, A. B.; Zhang, Q.; Porco, J. A. Jr. J. Am. Chem. Soc. 2011, 132, 13642. [link]
20. “Multidimensional Screening and Methodology Development for Condensations Involving Complex 1,2-Diketones,” Goodell, J. R.; Leng, B.; Snyder, T. K.; Beeler, A. B.; Porco, J. A. Jr. Synthesis, 2010, 2254. [link]
19. “Tandem Processes Identified from Reaction Screening: Nucleophilic Addition to Aryl N-Phosphinylimines Employing La(III)-TFAA Activation,” Kinoshita, H.; Ingham, Oscar, J.; Ong, W. W.; Beeler, A. B.; Porco, J. A., Jr. J. Am. Chem. Soc. 2010, 132, 6412. [link]
18. “A Time-Resolved Fluorescence–Resonance Energy Transfer Assay for Identifying Inhibitors of Hepatitis C Virus Core Dimerization,” Kota, S.; Scampavia, L.; Spicer, T.; Beeler, A. B.; Takahashi, V.; Snyder, J. K.; Porco, J. A., Jr.; Hodder, P.; Strosberg, A. D. Assay Drug Dev. 2010, 8, 96. [link]
17. “Development of an Automated Microfluidic Reaction Platform for Multidimensional Screening: Reaction Discovery Employing Bicyclo[3.2.1]octanoid Scaffolds.” Goodell J. R.; McMullen, J. P; Zaborenko, N.; Maloney, J. R.; C-X Ho; Jensen, K. F.; Porco, J. A. Jr.; Beeler, A. B. J. Org. Chem. 2009, 74, 6169. [link]
16. “Reaction Discovery Employing Macrocycles: Transannular Cyclizations of Macrocyclic Bis-lactams,” Han, C.; Rangarajan, S.; Voukides, A. C.; Beeler, A. B.; Johnson, R.; Porco, J. A., Jr. Org. Lett., 2009, 11, 413–416. [link]
15. “Library Synthesis Using 5,6,7,8-Tetrahydro-1,6-naphthyridines as Scaffolds,” Zhou, Y.; Beeler, A. B.; Cho, S.; Wang, Y.; Franzblau, S. G.; Snyder, J. K. J. Comb. Chem. 2008, 10, 534–540. [link]
14. “Identification of novel epoxide inhibitors of hepatitis C virus replication using a high-throughput screen,” Peng, L. F.; Kim, S. S.; Matchacheep, S.; Lei, X.; Su, S.; Lin, W.; Runguphan, W.; Choe, W-H; Sakamoto, N.; Ikeda, M.; Kato, N.; Beeler, A. B.; Porco, J. A. Jr.; Schreiber, S. L.; Chung, R. T. Antimicrobi. Agents Chemo. 2007, 10, 3756. [link]
13. “Nucleophilic addition to N-phosphinylimines by rare-earth-metal triflate/trifluoroacetic anhydride activation,” Winnie W. Ong; Aaron B. Beeler; Sarathy, Kesavan; James S. Panek; John A. Porco Angew. Chem. Int. Ed. 2007, 39, 7470. [link]
12. “1,2,3,4-Tetrahydro-1,5-naphthyridines and related heterocyclic scaffolds: exploration of suitable chemistry for library development,”Grace H. C. Woo; Aaron B. Beeler; John K. Snyder Tetrahedron 2007, 63, 5649. [link]
11. “Generation of Oxamic Acid Libraries: Antimalarials and Inhibitors of Plasmodium falciparum Lactate Dehydrogenase,” Choi, S-R.; Beeler, A. B.; Pradhan, A.; Watkins, E. B.; Rimoldi, J. M.; Tekwani, B.; Avery, M. A. J. Comb. Chem. 2007, 9, 292. [link]
10. “Discovery of Chemical Reactions through Multidimensional Screening,” Beeler, A. B.; Su, S.; Singleton, C. A.; Porco, J. A. Jr. J. Am. Chem. Soc. 2007, 129,1413. [link]
9. “Synthesis of 1,4,5-trisubstituted-1,2,3-triazoles by copper-catalyzed cycloaddition-coupling of azides and terminal alkynes,” Gerard, B; Ryan, J.; Beeler, A. B.; Porco, J. A Jr. Tetrahedron, 2006, 62, 6405. [link]
8. “Convergent Synthesis of Complex Diketopiperazines Derived from Pipecolic Acid Scaffolds and Parallel Screening against GPCR Targets,” Dandapani, S.; Lan, P.; Beeler, A. B.; Beischel, S.; Abbas, A.; Roth, B. L.; Porco, J. A. Jr.; Panek, J. S. J. Org. Chem. 2006, 71, 8934.[link]
7. “Synthesis of a Library of Complex Macrodiolides Employing Cyclodimerization of Hydroxy Esters,” Beeler, A. B.; Acquilano, D. E.; Su, Q.; Yan, F.; Roth, B. L.; Panek, J. S.; Porco, J. A. Jr. J. Comb. Chem. 2005, 7, 673. [link]
6. “Chemical Library Synthesis Using Convergent Approaches,” Beeler, A. B.; Schaus, S. E.; Porco, J. A. Jr. Curr. Opin. Chem. Bio. 2005, 9, 277. [link]
5. “Convergent Synthesis of a Complex Oxime Library Using Chemical Domain Shuffling,” Su S.; Eastwood, E. L.; Beeler, A. B.; Yeager, A. R.; Lan, P.; Arumugasamy, J.; Acquilano, D. E.; Min, G. K.; Giguere, J. R.; Lei, X.; Zhou, Y.; Panek, J. S.; Snyder, J. K.; Schaus, S. E.; Porco, J. A. Jr. Org. Lett. 2005, 9, 2751. [link]
4. “Synthesis and In-Vitro Biological Evaluation of Fluorosubstituted-4-Phenyl-1,2,3,6-Tetrahydropyridines as Monoamine Oxidase B Substrates,” Beeler, A. B.; Gadepalli, R. S.; Steyn, S.; Castagnoli, N. Jr.; Rimoldi, J. M. Biorg. Med. Chem. 2003, 11, 5229-5234. [link]
3. “Stereochemical Diversity Through Cyclodimerization: Synthesis of Polyketide-like Macrodiolides,” Su, Q.; Beeler, A. B.; Lobkovsky, E.; Porco, J. Jr.; Panek, J. Org. Lett. 2003, 5, 2149-2152. [link]
2. “Toxicity of Fipronil and Its Degradation Products in Procambarus sp.: Field and Laboratory Studies,” Schlenk, D.; Huggett, D. B.; Allgood, J.; Bennett, E.; Rimoldi, J.; Beeler, A. B.; Block, D.; Holder, A. W.; Hovinga, R.; Bedient, P.Arch. Environ. Contam. Toxicol. 2001, 41, 325-332. [link]
1. “Synthesis of fipronil sulfide, an active metabolite from the parent insecticide fipronil,” Beeler, A. B.; Schlenk, D. K.; Rimoldi, J. M. Tetrahedron Lett. 2001, 42, 5371-5372